 How are Radiation and Radioactivity Different? How are Radiation and Radioactivity Different?
 Using an open fire as an example, if the fire represents radioactivity, then the heat emitted from the fire would be the radiation. Using an open fire as an example, if the fire represents radioactivity, then the heat emitted from the fire would be the radiation.
Differences among radiation, radioactivity, and radioactive material
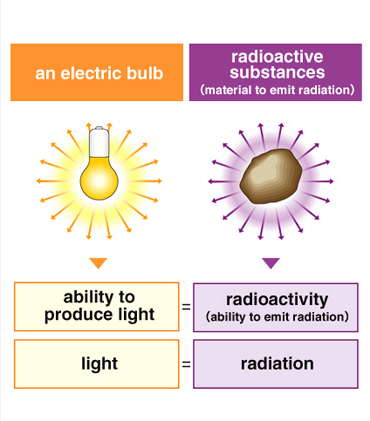 |
Radiation takes many different forms, including X-rays and alpha particles. These forms of radiation can pass through matter and expose photographic film. However, radioactivity indicates the capacity of a material to emit radiation. Therefore, in the example of the fire, the fire represents the "radioactivity" and the heat emitted by the fire is equivalent to the "radiation". Coffee and its aroma can be used as another example. However, because radiation and radioactivity cannot be seen, sensed, or smelled, in reality there is no exact equivalent we can use as an example. Just remember that the definition of radioactivity is "the property or capacity to emit radiation".
Originally, the term radioactivity was coined by Madame Marie Curie, back when she was investigating whether any substances other than the already-discovered uranium could produce radiation. While doing this, she discovered that thorium also could.
Because the property of emitting radiation was not limited to uranium, she used the term "radioactivity" to more universally describe the "property of a particular substance to emit radiation". She subsequently identified substances with far more intense radioactivity than uranium or thorium. By separating, concentrating, and purifying intensely radioactive substances, she also discovered polonium and radium. To date, more than 70 naturally occurring radioactive substances, known as isotopes, have been discovered. The types of radiation that radioisotopes can emit include alpha, beta, gamma, and neutron radiation. X-rays are also a form of radiation, but we do not say that a machine that generates X-rays is radioactive.
The unit for measuring radioactivity, or the amount of radiation (a substance) can emit, is the becquerel. When a radioisotope emits radiation, the atomic nucleus breaks down naturally and transforms, producing radiation in the form of alpha or beta particles. This phenomenon is known as radioactive decay. The unit of radioactivity, the becquerel, counts the number of transformations occurring in one second. Expressing radioactivity in becquerels allows the weight of a particular radioisotope to be ascertained. Long ago, the curie was used, in honor of Pierre and Marie Curie. The curie represents the radioactivity of 1 gram of radium, a very large number equivalent to 3.7 ×101o becquerels, or disintegrations per second.
References:
Hoshano Q&A (Radiation Q&A): S. Yamashita (ed.), Nagasaki Prefecture Atomic Bomb Survivors Support Division
Hoshano Monogatari ! to ? no Hazama de (The Story of Radioactivity: Between ! and ? ), T. Kinugasa (ed.), Iryo Kagakusho
Checked by Kuniaki Hayashi M.D. Professor Nagasaki University Graduate School of Biomedical Sciences
Checked by Yutaka Okumura Professor Nagasaki Atomic Bomb Disease Institute Nagasaki University Graduate School of Biomedical Sciences
Written by Kumio Okaichi Ph.D. Associate Professor Nagasaki Atomic Bomb Disease Institute Nagasaki University Graduate School of Biomedical Sciences
|

